
Understanding Degradation of Capacitive Deionization Cells
1. Motivation
Capacitive deionization (CDI) is a promising, porous-electrode–based desalination technology [1, 2]. The primary mechanism responsible for ion storage in CDI is typically electrosorption: the electrostatically driven accumulation of ions in the electric double layer (EDL) near the interface between aqueous and charged phases. Recent reviews have highlighted long–term performance degradation of CDI cells due to electrochemical reactions at carbon electrode surfaces [2, 3] – specifically, the oxidation of carbon sites on the anode.
In this study, we introduce a comprehensive model for long–term, oxidation–induced performance degradation in flow-through-electrode CDI, and we share the numerical methods required for full-cell, multi-cycle simulations. The results reveal that oxidation and surface group protonation/deprotonation occur in a spatially and temporally inhomogeneous fashion as a result of coupled reaction–transport dynamics.
2. Porous Electrode Theory
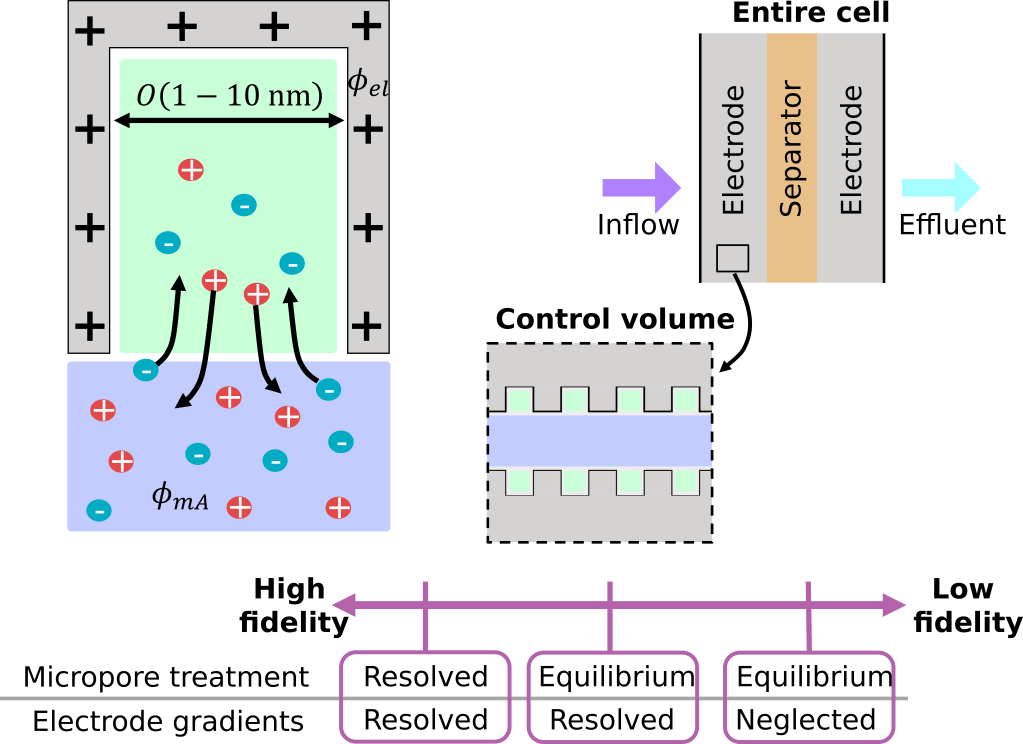
In a flow-through-electrode (FTE) CDI cell, electrolyte flows through a stack of porous electrodes with a hierarchical pore structure, wherein macropores are large enough to permit flow of electrolyte through the porous medium while micropores provide space for electrostatically-induced storage of ionic species when the electrodes are charged. The governing equations for transport at the micropore scale are well known, but simulations that resolve this scale would prove too expensive to capture ionic concentration and electric potential fields across the entire cell. The key to overcoming this dilemma, as described originally by Johnson and Newman [4], is recognition that the time scale of pore–scale dynamics is much faster than the time scale of device-scale transport. This analysis implies that micropores are in local quasi-steady equilibrium with their surroundings, allowing construction of a continuum model with effective control volumes that include numerous macropores and micropores in local equilibrium.
3. Electrode Degradation Surface Chemistry
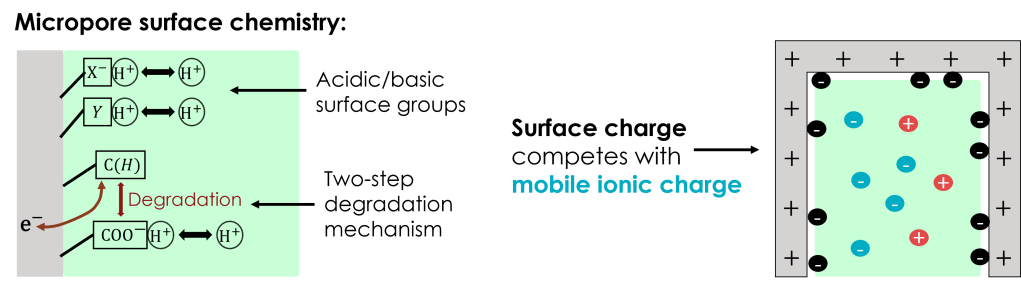
The presence of acidic and basic surface groups is expected to play a substantial role in the micropore dynamics. These groups may contribute to surface charge, depending on equilibration with the local pH via protonation/de-protonation reactions. For the case studied here, weakly acidic carboxylic groups are formed through electron-transferring degradation reactions. These carboxylic groups may de-protonate in sufficiently basic conditions, contributing to negative surface charge and competing against electrosorption of mobile anions.
4. In-house Numerical Solver
We develop an in-house numerical solver for the governing equations, as described thoroughly in the associated publication. Second-order central differences are used for spatial discretization, and a third-order, implicit, multi-step method is used for temporal discretization. Additionally, we incorporate adaptive time-step refinement, since rapid transients must be resolved each time the voltage signal flips from charging to discharging or vice-versa. This combination of methods permits full-cell simulations over multiple cycles, even in a simple MatLab implementation.
5. (Numerical) Electrochemical Impedance Spectroscopy
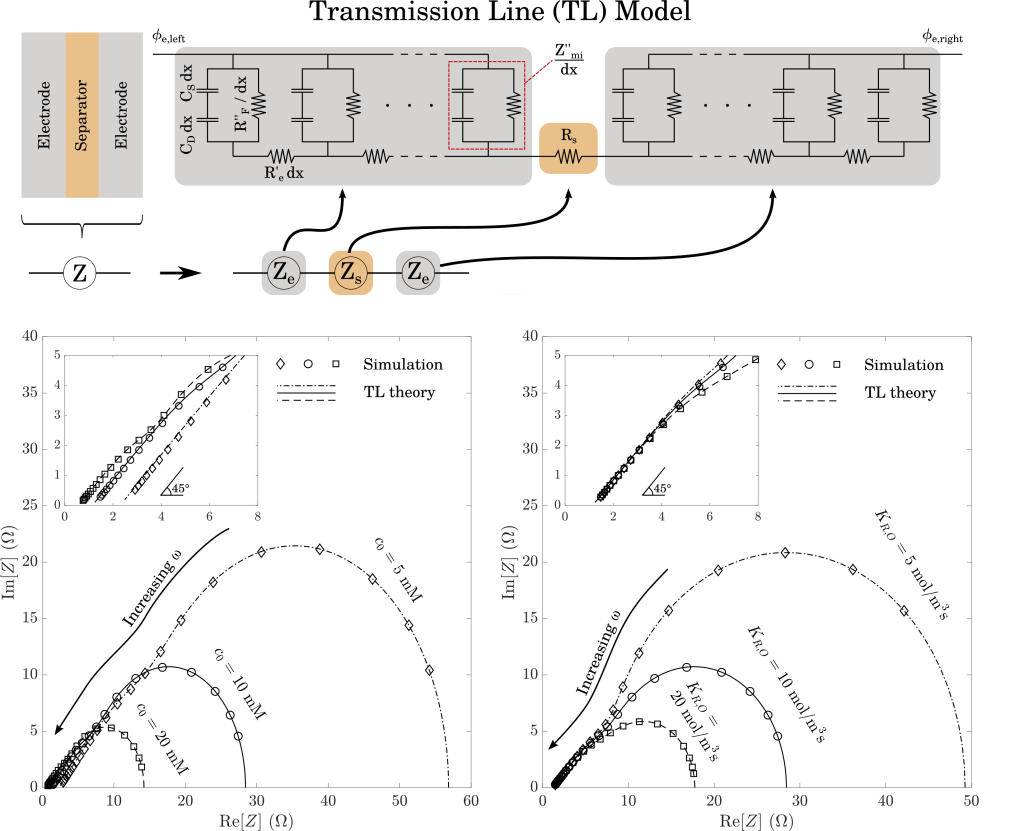
We verify consistency of the solver by comparing results against an analytical “transmission-line” model, which is valid in the limit of small voltage perturbations. Agreement is expected if the numerical solver has been implemented correctly and driving voltage perturbations are sufficiently small. As shown here, we find excellent agreement across the full range of bulk electrolyte concentrations and Faradaic reaction rate coefficients.
6. CDI Degradation Examined
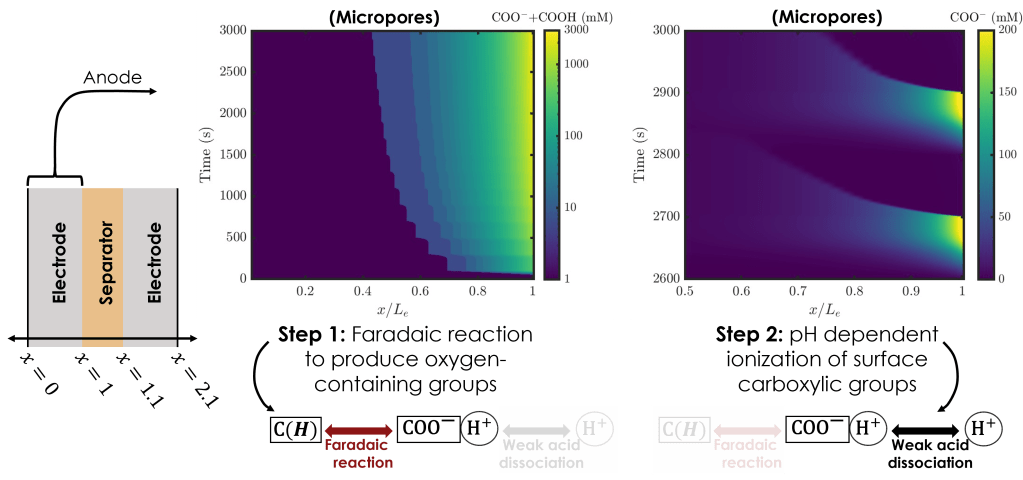

Full-cell simulations reveal that the dynamics of cell charging and discharging exhibit substantial inhomogeneity in space and in time across the electrodes, with propagating, shock–like pH fronts that transiently shift the local micropore conditions and strongly impact interfacial reaction rates. Ultimately, these features would not have been observable without a modeling approach that captured the O(1 nm) micropores accurately (here, analytically) while allowing simulation of the entire deionization cell (thickness of O(1 mm)).
References
[1] Porada, S. et al., Progress in Materials Science 58, 1388–1442, 2013
[2] Suss, M. E. et al., Energy and Environmental Science 8, 2296–2319, 2015
[3] Zhang, C. et al., Water Research 128, 314–330, 2018
[4] Johnson, A.M. and Newman, J., J. Electrochem. Soc. 118, 510–517, 1971
Acknowledgements
Financial support from the Stephen and Nancy Grand Technion Energy Program (GTEP) is acknowledged. Simulations were partially performed on the Armstrong and Shepard clusters at the Stanford High Performance Computing Center.
Material on this page was published as a journal article in Desalination. The full citation for the article is:
A. Balaji-Wright, J. Wu, A. N. Shocron, A. G. Dana, M. Suss, and A. Mani, “Understanding degradation of capacitive deionization cells: Full–cell simulations with anode corrosion,” Desalination, vol. 587, p. 117924, 2024.